Abstract
Introduction: In a single blood donation a donor loses approximately 250 mg of iron. Serum ferritin is an accepted and widely used method for determining iron status and is used to assess the iron status of all new and selected active blood donors in the Blood Bank in Iceland. The World Health Organization defines depleted iron stores as ferritin <15 μg/l, but at our institution the ferritin cut-off for active blood donors has been >8 μg/l. Where ferritin assays are used to determine donor eligibility status, the accuracy of these assays is critical. We have noticed discrepancies between the results of paired ferritin measurements on different ferritin immunoassay platforms, both between local laboratories and on external control reports.
Objective: The objectives of this study were 1) to examine the iron status of Icelandic blood donors and assess the frequency of iron deficiency in new and active donors, in both genders and different age groups, 2) to compare serum ferritin measurements between two laboratories using different platforms, and 3) to assess the potential impact of using different platforms on donor deferral rates.
Methods: A retrospective study of blood donor ferritin results was performed in the Blood Bank for the calendar years 2015 and 2016. Blood donor data were obtained through the laboratory information system (ProSang®). For comparison of ferritin measurements between different platforms 106 blood samples from blood donors were obtained and ferritin measured both at the Blood Bank (Vitros®) and the hematology laboratory at Landspítali (Cobas®). Both platforms are based on the same technique which is a two-step sandwich ELISA. Data was analyzed using Excel and the R program.
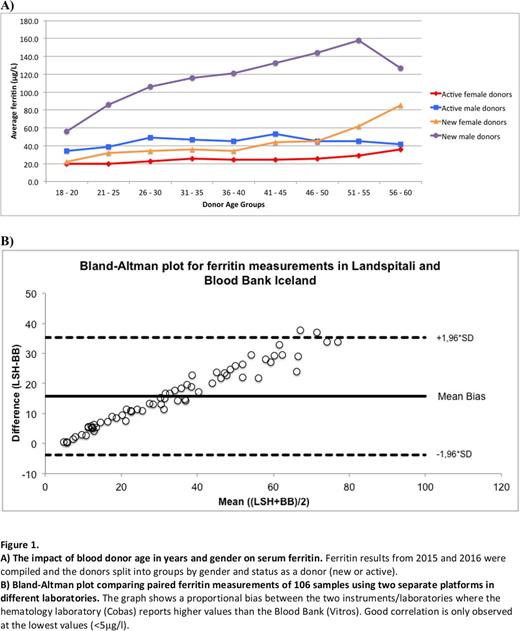
Results: The median ferritin value for new blood donors was 51 μg/l (n = 3529); more specifically 86.4 μg/l for men (n = 1824) and 26.6 μg/l for women (n = 1705). The frequency of iron deficiency (ferritin <15μg/l) among new blood donors was 11%; 1% in men and 22% in women. Ferritin results were available for approximately 15% of active blood donors. The median ferritin value for active donors was 27.8 μg/l (n = 3223); more specifically 32.9 μg/l for men (n = 2159) and 20.8 μg/l for women (n = 1064). Among new donors ferritin levels were on average lowest in the youngest age groups for both genders, but increased with higher age, gradually among men and after age 40 among women (figure 1A). Donor age had less impact on ferritin levels in active blood donors. A proportional bias was observed between ferritin measurements for the two different instruments/laboratories (figure 1B). Good correlation was only observed between measurements at the lowest values (<5 μg/l) but correlation decreased with higher ferritin values. As an example a single donor sample gave ferritin values of 9.91 μg/l using Vitros but 15 μg/l using the Cobas. Of the 106 donors providing samples for paired measurements, 9% would be deferred as blood donors due to low ferritin (<15 μg/l) using the Vitros assay whereas only 2% would be deferred using the Cobas analyser. As the Vitros consistently gave lower ferritin values, using this instrument in the Blood Bank will lead to increased donor deferral rates due to low ferritin compared to the Cobas.
Conclusion: One fourth of newly registered female blood donors were iron deficient. It is important to monitor ferritin in blood donors to prevent the development of iron deficiency. A prospective study of iron stores in active donors is needed to establish the prevalence of iron deficiency in this population. The proportional bias observed between the two instruments/laboratories for ferritin assays underlines the importance of taking instrument-related performance into account when establishing ferritin cut-offs for blood donor deferral. Using a more "sensitive" assay will lead to fewer donor deferrals due to iron deficiency but may cause increased deferrals due to high ferritin levels.
No relevant conflicts of interest to declare.
Author notes
Asterisk with author names denotes non-ASH members.

This feature is available to Subscribers Only
Sign In or Create an Account Close Modal